Dynamical Systems
&
Neuroendocrinology
Friday, October 16th, 2009, Paris
The registration is free but mandatory. If you intend
to participate in the workshop,
please send an e-mail to: alexandre.vidal
[at] univ-evry.fr
Note: The talk of Dr. Olivier Kah is
cancelled.
____________________________________________
Speakers
and abstracts
Richard BERTRAM
Alfredo
ULLOA-AGUIRRE
Olivier KAH
Krasimira
TSANEVA-ATANASOVA
Gareth LENG
Jacques YOUNG
Prof.
Richard Bertram
Department
of Mathematics and Programs in Neuroscience and Molecular Biophysics,
Florida
State University,
Tallahassee,
Florida.
A Mathematical Study of Electrical Bursting in Pituitary Cells.
Pituitary
lactotrophs, somatotrophs, and corticotrophs often
exhibit electrical bursting patterns, consisting of periodic episodes of
electrical impulses followed by periods of quiescence. Unlike bursting observed
in nerve cells and pancreatic islets, the impulses in pituitary bursts have a
small amplitude, and the burst frequency is high. Mathematical models of
bursting are typically analyzed using geometric singular perturbation analysis,
often called fast/slow analysis. This analysis has been applied to pituitary
bursting and contrasted with square wave bursting, a typical type of bursting
in neurons and pancreatic islets. The analysis highlights the many differences
in the dynamics of these two forms of bursting. In this talk, we describe these
dynamics and demonstrate that, although the two seem very different, it is
possible to transform one to the other through variation of a single parameter.
Moreover, parameters that can achieve this transformation are biologically
plastic, so it is reasonable that they could vary from one cell type to
another.
Prof.
Alfredo Ulloa-Aguirre,
Research
Unit in Reproductive Medicine,
Instituto
Mexicano del Seguro Social,
Mexico
D.F., Mexico.
Invited
STUDIUM Professor at INRA Tours, France.
GnRH
resistance and congenital hypogonadotropic hypogonadism in humans: A GPCR
conformational disease.
The mammalian gonadotropin-releasing
hormone receptor (GnRHR) belongs to the superfamily of G-protein coupled receptors, specifically
the family related to the rhodopsin- and b2-adrenergic-like receptors. Unlike other
members of the GPCRs superfamily,
the GnRHR exhibits several unique futures, including
the lack of the carboxyl-terminal extension into the cytosol
and, in the case of primate GnRHRs, the presence of Lys at position 191 in the second extracellular
loop, which restricts cell surface plasma membrane expression of the receptor
by hindering formation of the Cys14-Cys200 disulfide bridge, which is necessary
to stabilize the receptor in a conformation compatible with endoplasmic
reticulum export.
Point
mutations in cell surface receptors may result in the production of misfolded proteins that are translated but do not reach
their proper destination in the cell. This is the case of loss-of-function
mutations in the human GnRHR, which are a rare cause
of hypogonadotropic hypogonadism
in humans, a disease leading to reproductive failure due to partial or complete
inability of the pituitary gonadotrops to respond to
agonist. The majority of these mutant GnRHRs are
trafficking-defective receptor proteins, whose function can be restored in vitro by genetic or pharmacologic
means. We have recently applied a combined strategy (mutagenesis and functional
studies as well as computational modeling and molecular dynamics simulations)
to analyze some structure-function relationships of the human GnRHR and the mechanism(s) whereby mutations lead to misfolded receptor proteins. In this talk I will describe
how application of these strategies have contributed to elucidate the
conformational effects of Lys191 in the human GnRHR
and the role of the Cys14-Cys200 disulfide bridge in receptor cell surface
plasma membrane expression.
Dr.
Olivier Kah, CNRS Research Director
Neurogenesis And Oestrogens,
University
of Rennes 1, UMR CNRS 6026,
Rennes,
France.
GnRHs and their receptors in Metazoa: From multiple
to highly-specialized functions.
Over
the last 40 years, the GnRH decapeptides and their
receptors have been the topic of a constantly-renewed interest due to their key
roles in the central control of ovulation in vertebrates. Studies in a growing
number of invertebrate species have now led to the view that this
ligand/receptor pair has emerged very early in evolution, some 650 millions
years ago, and has progressively specialized in the control of synthesis and
release of gonadotropins. This evolution was
accompanied by a number of gene duplications and gene losses providing some
insights in the history of this peptide family although many questions remain
open for future studies.
Dr.
Krasimira Tsaneva-Atanasova
Department
of Engineering Mathematics,
University
of Bristol,
Bristol,
United Kingdom.
A Mathematical Model for Regulation of Gonadotrophins
Secretion.
Gonadotrophin-releasing
hormone (GnRH) is a hormone released from the brain to control the secretion of
reproductive hormones. Like many other chemical messages it is released in
brief pulses. Pulsatile GnRH can increase fertility (e.g. in IVF programmes)
whereas sustained GnRH reduces fertility (and is used to treat
hormone-dependent cancer) but the ways in which the GnRH receptor and its
intracellular signalling cascade decode these kinetic aspects of stimulation
are essentially unknown. Given that clinical use of GnRH agonists relies on
avoidance or exploitation of this effect, it is remarkable how little is known
about the way gonadotrophs decode stimulus kinetics.
In this talk we present a biophysical model of the key players that govern GnRH
signalling. The model results are closely related to experimental data.
Prof.
Gareth Leng
School of
Biomedical Sciences,
University
of Edinburgh, College of Medicine and Veterinary Sciences,
Edinburgh,
United Kingdom.
Modelling neuroendocrine
systems.
Peptides
in the hypothalamus are not like conventional neurotransmitters; their release
is not particularly associated with synapses, and their long half-lives mean
that they can diffuse to distant targets. Peptides can act on their cells of
origin to facilitate the development of patterned electrical activity, they can
act on their neighbours to bind the collective activity of a neural population
into a coherent signalling entity, and the co-ordinated population output can
transmit waves of peptide secretion that act as a patterned hormonal analogue
signal within the brain. At their distant targets, peptides can re-programme
neural networks, by effects on gene expression, synaptogenesis, and by
functionally rewiring connections by priming activity-dependent release.
My
lab has studied mainly the oxytocin and vasopressin
neurones of the hypothalamus, these neurones fire in distinctive patterns that
govern and in turn are governed by the peptide secretion that they induce. Oxytocin cells display remarkable synchronised bursts that
arise through emergent properties of an interactive network; vasopressin cells
also burst, but asynchronously in a very different way and for very different
reasons. In their different ways, these two neuronal systems have become
important model systems in neuroscience; in this talk I will talk about
modelling these model systems.
Prof. Jacques Young
Service d’Endocrinologie et des Maladies de la Reproduction, INSERM U 693,
Université Paris Sud-11, APHP, CHU de Bicêtre,
Paris,
France.
Pulsatile GnRH secretion in human physiology
and pathology.
GnRH is the central regulator of the
reproductive hormonal cascade and was first isolated from mammalian hypothalami
as the decapeptide (pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly.NH2).
GnRH is processed in hypothalamic neurons from a precursor polypeptide by enzymic processing and packaged in storage granules that
are transported down axons to the external zone of the median eminence. The
peptide is released in synchronized pulses from the nerve endings of about 1000
neurons into the hypophyseal portal system every 30–120 min to stimulate the
biosynthesis and secretion of LH and FSH from pituitary gonadotropes.
Each GnRH pulse stimulates a pulse of LH release, but FSH pulses are less
distinct. The frequency of pulses is highest at the ovulatory LH surge and
lowest during the luteal phase of the ovarian cycle. The asynchronous patterns
of LH and FSH release result from changes in GnRH pulse frequency, modulating
effects of gonadal steroid and peptide hormones on
FSH and LH responses to GnRH, and differences in the half-lives of the two
hormones.
Low doses of
synthetic GnRH delivered in a pulsatile fashion to simulate the endogenous GnRH
levels in the portal vessels restore fertility in men and women with hypogonadotropic hypogonadism.
However, high doses of GnRH or agonist analogs
desensitize the gonadotrope with resultant decrease
in LH and FSH and a decline in ovarian and testicular function. This
desensitization phenomenon is extensively applied in clinical medicine for the
treatment of a wide range of diseases among which prostate cancer and
precocious puberty.
Isolated GnRH
deficiency is the clinical syndrome that results from failure of this normal
pattern of episodic GnRH secretion to occur. It is characterized by complete or
partial absence of any endogenous GnRH-induced LH pulsations and normalization
of pituitary and gonadal function in response to
physiological regimens of exogenous GnRH replacement.
Clinically, the
diagnosis of GnRH deficiency is made in adolescence when there is failure of
pubertal development and absence of appearance of secondary sex
characteristics. In isolated GnRH deficiency, a variety of aberrant gonadotropin secretory patterns have been observed,
indicating a spectrum of defects in GnRH secretion in keeping with the diverse
clinical presentation. These different pulsatile abnormalities will be
discussed.
____________________________________________
Schedule
Lecture Hall of the Jacques Monod Institute:
|
8:45 |
– |
9:15 |
Welcome |
|
|
9:15 |
– |
9:30 |
Introduction |
|
|
9:30 |
– |
10:30 |
Richard
Bertram |
A mathematical study of electrical bursting in pituitary cells |
|
10:30 |
– |
11:00 |
Break |
|
|
11:00 |
– |
12:00 |
Alfredo
Ulloa-Aguirre |
GnRH resistance and congenital hypogonadotropic hypogonadism in
humans: A GPCR conformational disease |
Lecture Hall Durand, Esclangon Building:
|
1:30 |
– |
2:30 |
Krasimira Tsaneva-Atanasova |
A mathematical model for
regulation of gonadotrophins
secretion |
|
2:30 |
– |
3:30 |
Gareth Leng |
Modelling neuroendocrine systems |
|
3:30 |
– |
4:00 |
Break |
|
|
4:00 |
– |
5:00 |
Jacques
Young |
Pulsatile
GnRH secretion in human physiology
and pathology |
|
5:00 |
– |
5:30 |
Workshop
Conclusion |
|
____________________________________________
Organizers
|
|
|||
|
|
|||
|
|
|
____________________________________________
Sponsors
ANAR (Analyse Non linéaire et Application aux Rythmes du vivant), funded by the ANR.
REGATE (REgulation of the
GonAdoTropE Axis), Large-Scale project INRIA.
____________________________________________
Participants
____________________________________________
Practical
information
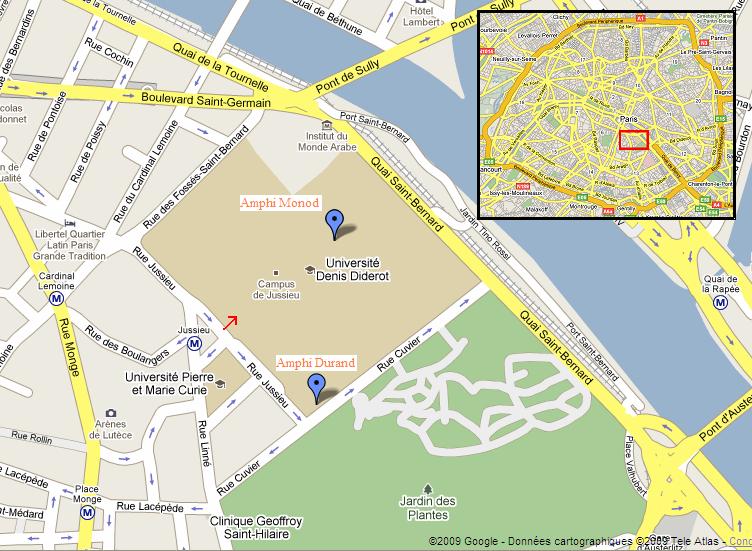
The
workshop will take place in the Jussieu Campus (Université Pierre & Marie Curie, UPMC, 4 place jussieu, Paris 5ème arr.), in the heart of Paris (see
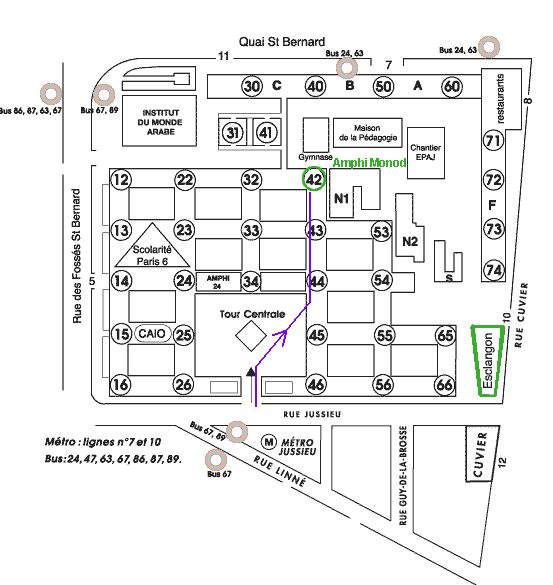
neighborhood map on the next page). It is accessible with the metro, station “Jussieu” on lines 7 and 10, and many bus lines (see the
Campus schema below).
During
the morning, the workshop will take place in the lecture hall of the Jacques Monod Institute, Tower 42 (level -1). Then, during the
afternoon, the workshop will continue in the lecture hall Durand in the Esclangon Building.
The
registration is free but mandatory. If you intend to participate in the
workshop, please send an e-mail to: alexandre.vidal
[at] univ-evry.fr
Useful
links:
____________________________________________
